Research Symposium
23rd annual Undergraduate Research Symposium, April 6, 2023
Ziya Tian Poster Session 4: 4:00 pm - 5:00 pm/ Poster #412
BIO
I grew up in Beijing, China, and moved to Tallahassee in 2018. At FSU, I am a second-year undergraduate student majoring in biological science and minoring in chemistry, computer science, and mathematics. After college, I plan to go to graduate school to pursue a Ph.D. Currently, my research interests include the structures and interactions of molecules in biological systems, but I am always excited to learn more about the fascinating science of life.
Modeling the Interaction Between Dengue Virus NS2B3 and Human cGAS
Authors: Ziya Tian, Qian YinStudent Major: Biological Science
Mentor: Qian Yin
Mentor's Department: Biological Science Mentor's College: College of Arts and Sciences Co-Presenters: Varshil Nunna
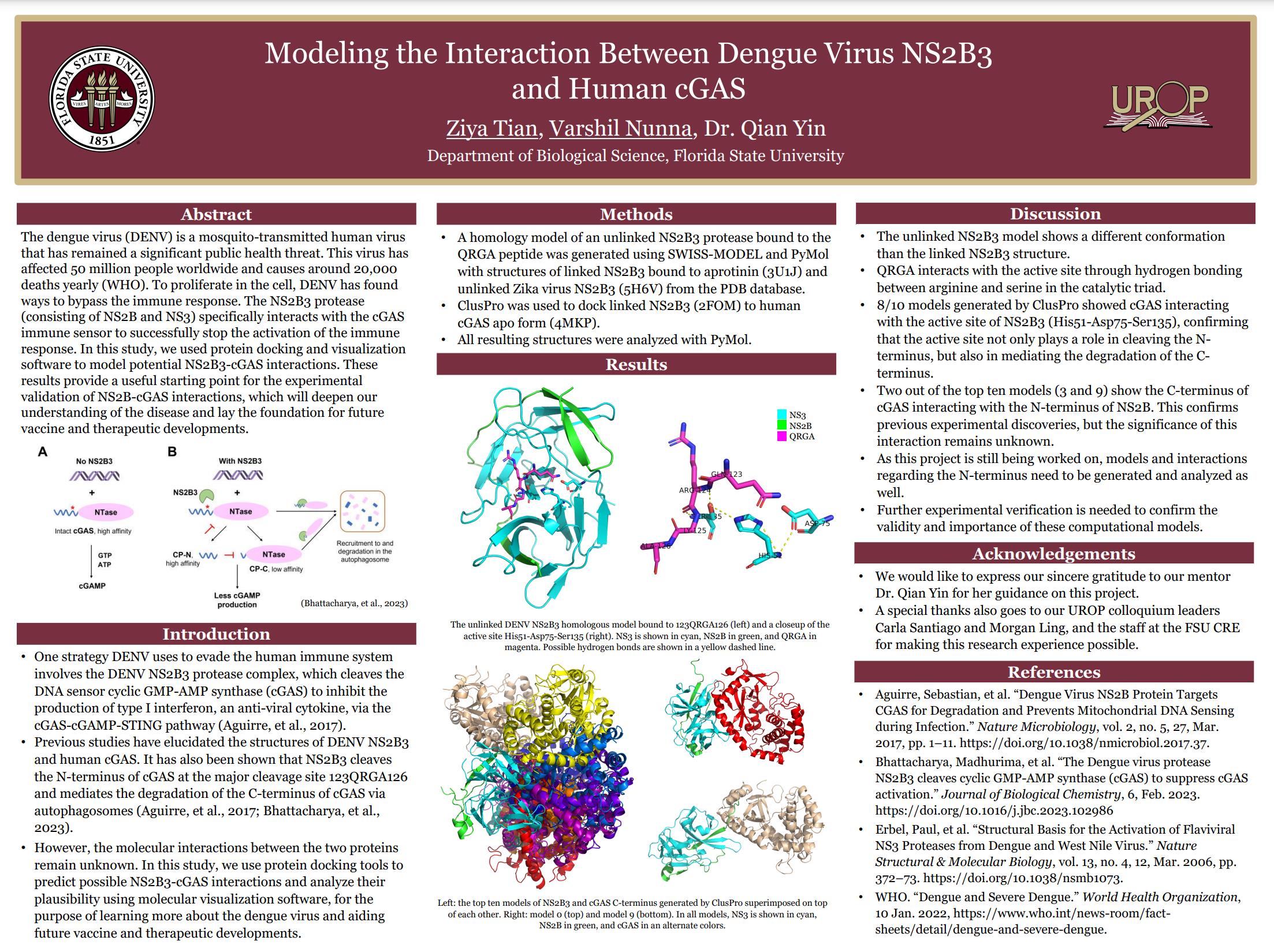
Abstract
The dengue virus (DENV) is a mosquito-transmitted human virus that has remained a significant public health threat. This virus has affected 50 million people worldwide and causes around 20,000 deaths yearly. To proliferate in the cell, DENV has found ways to bypass the immune response. The NS2B3 protease (consisting of NS2B and NS3) specifically interacts with the cGAS immune sensor to successfully stop the activation of the immune response. Previous studies have elucidated the structures of both NS2B3 and human cGAS. It has also been shown that NS2B3 cleaves the N-terminus of cGAS and mediates the degradation of the C-terminus of cGAS via autophagosomes. However, the molecular interactions between the two proteins remain unknown. In this study, we used protein docking and visualization software to model potential NS2B3-cGAS interactions. Specifically, we analyzed similar published structures and manipulated a peptide-bound NS2B3 model to show a possible interaction between the active site of NS2B3 and N-terminus cleavage sites on cGAS. For the interaction between NS2B3 and the C-terminus of cGAS, we used the ClusPro protein docking server to generate ten models and found that cGAS potentially binds to both the active site of NS2B3 and the N-terminal tail of NS2B. These results provide a useful starting point for the experimental validation of NS2B-cGAS interactions, which will deepen our understanding of the disease and lay the foundation for future vaccine and therapeutic developments.
Keywords: biology, immunology, virus, protein, structure