Research Symposium
23rd annual Undergraduate Research Symposium, April 6, 2023
Julian Bazo Poster Session 3: 2:45 pm - 3:45 pm/ Poster #169

BIO
Hello, my name is Julian Bazo and I am a fourth-year student from Coral Springs, FL. I've been working in the Lazenby Lab for almost two semesters, focusing on characterizing iron-nickel nano carbide electrocatalysts for water-splitting applications. I am graduating this coming spring with a bachelor's degree in biochemistry, minoring in chemistry, biology, and physics. I've very much enjoyed my time and FSU and I'm looking forward to presenting my research at the URS 2023.
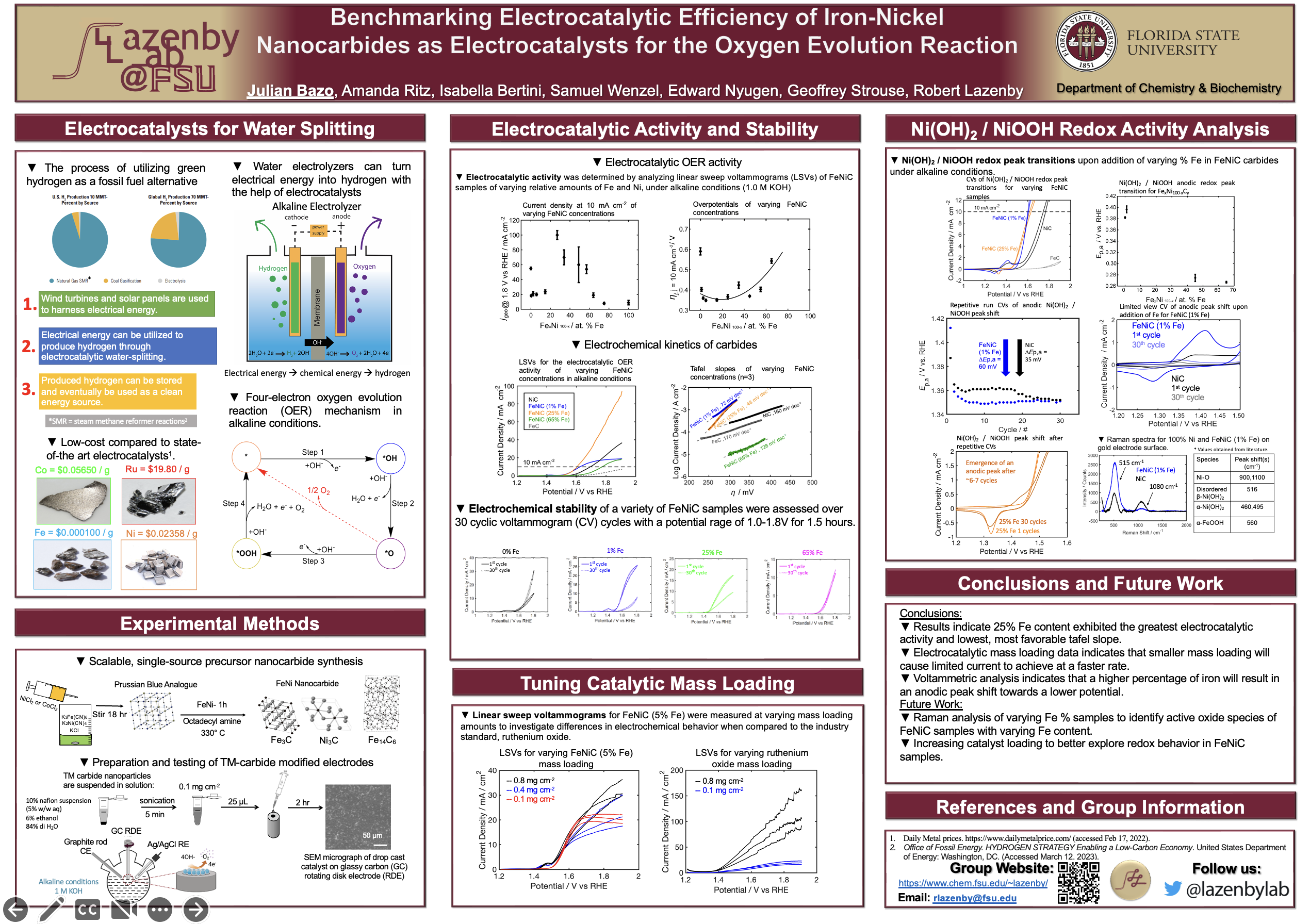
Benchmarking Electrocatalytic Efficiency of Iron-Nickel Nanocarbides as Electrocatalysts for the Oxygen Evolution Reaction
Authors: Julian Bazo, Amanda RitzStudent Major: Biochemistry
Mentor: Amanda Ritz
Mentor's Department: Chemistry & Biochemistry Mentor's College: College of Arts & Sciences Co-Presenters:
Abstract
Electrocatalytic water-splitting, the process of turning electrical energy into chemical energy aids in the production of clean hydrogen fuel for energy storage applications. The kinetically sluggish oxygen evolution reaction (OER) reaction is essential for water splitting electrolysis and can often hinder the efficient production of hydrogen. For the OER to occur, a large energy requirement must be fulfilled. Electrocatalysts are used in order to lower the amount of energy needed for electrocatalytic OER and the overall water-splitting reaction. When looking for a viable OER electrocatalyst it is important to achieve high electrocatalytic activity and other characteristics such as, long-term stability, low cost, and efficient product scalability. Iron-Nickel (FeNi)-Prussian blue analogues (PBA) as well as metal organic frameworks (MOFs) allow for the low-cost and scalable production of bimetallic FeNi nanocarbides with tunable Fe:Ni ratios. In this work, we assessed the effects of catalytic mass loading using voltametric methods and analyzed the effects of short-term exposure to OER under alkaline conditions on oxidation of material and changes in redox peaks. The formation of a Ni(OH)2/NiOOH redox couple has been previously identified in commonly synthesized FeNi oxyhydroxide catalysts, however our work identifies a shift in redox potential during repeated cyclic voltammetry measurements. We aim to demonstrate the importance of the active redox state in Ni for the electrocatalytic activity of FeNi nanocarbides.
Keywords: Electrochemistry, Oxygen Evolution Reaction, Green energy, Electrocatalytic Water-Splitting, Nano-materials