Research Symposium
25th annual Undergraduate Research Symposium, April 1, 2025
Samuel Won Poster Session 4: 3:00 pm - 4:00 pm / Poster #272

BIO
I am a sophomore studying biological sciences with plans to minor in chemistry and computer science. I love running, tinkering around in the garage, and playing with my friends in my free time. I am from Vancouver, Washington. I am hoping to attend medical school to be a orthopedic surgeon and eventually a flight surgeon for NASA.
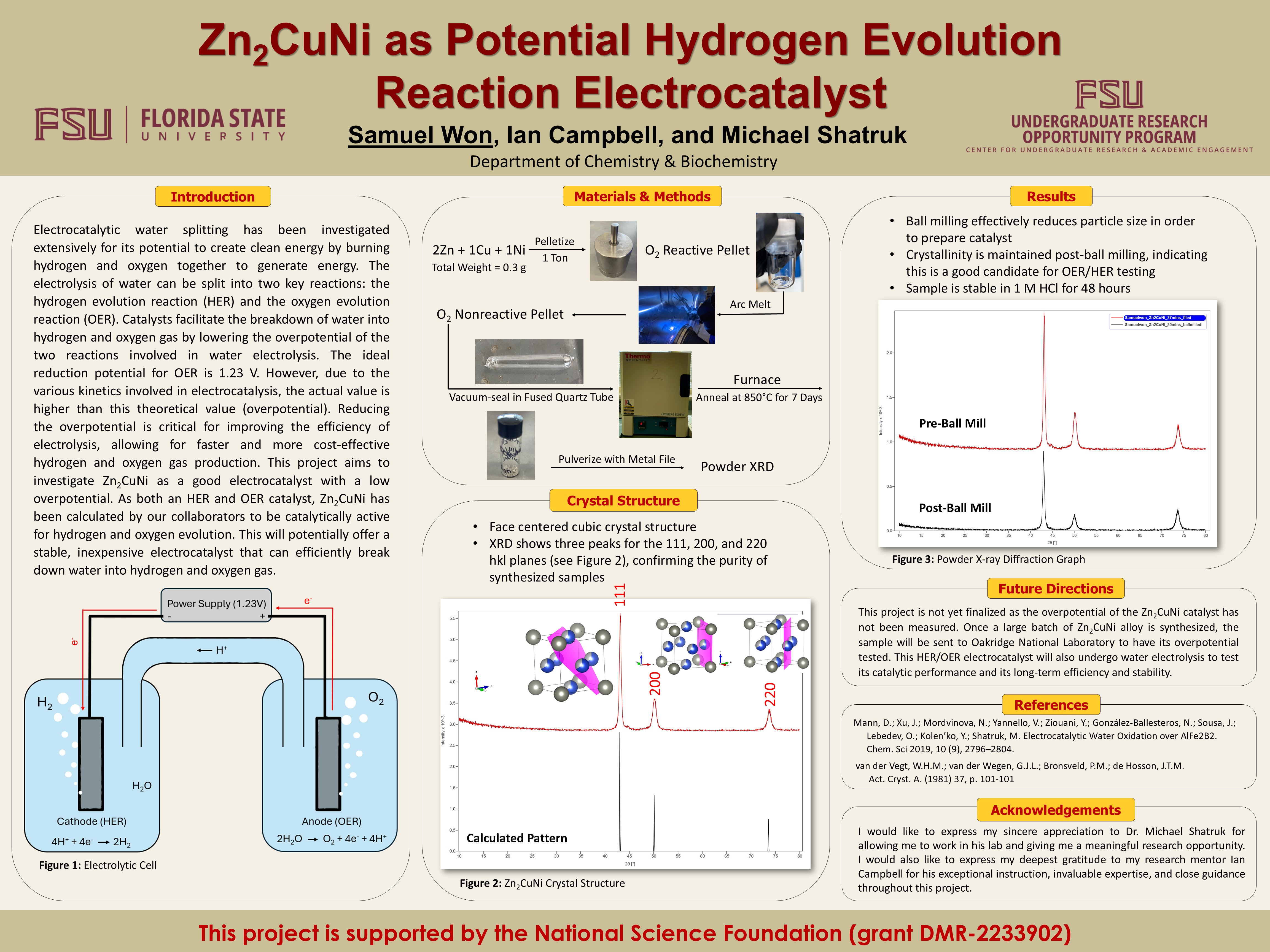
Zn2CuNi as Potential Hydrogen Evolution Reaction Electrocatalyst
Authors: Samuel Won, Michael ShatrukStudent Major: Biological Sciences
Mentor: Michael Shatruk
Mentor's Department: Department of Chemistry and Biochemistry Mentor's College: College of Arts and Sciences Co-Presenters:
Abstract
Electrocatalytic water splitting has been investigated extensively for its potential to create clean energy by burning hydrogen and oxygen together to generate energy. The electrolysis of water can be split into two key reactions: the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). Catalysts facilitate the breakdown of water into hydrogen and oxygen gas by lowering the overpotential of the two reactions involved in water electrolysis. The ideal reduction potential for OER is 1.23 V. However, due to the various kinetics involved in electrocatalysis, the actual value is higher than this theoretical value (overpotential). Reducing the overpotential is critical for improving the efficiency of electrolysis, allowing for faster and more cost-effective hydrogen and oxygen gas production. This project aims to investigate Zn2CuNi as a good electrocatalyst with a low overpotential. As both an HER and OER catalyst, Zn2CuNi has been calculated by our collaborators to be catalytically active for hydrogen and oxygen evolution. This will potentially offer a stable, inexpensive electrocatalyst that can efficiently break down water into hydrogen and oxygen gas.
Keywords: chemistry, catalyst, water