Research Symposium
25th annual Undergraduate Research Symposium, April 1, 2025
Alexandra Martin Poster Session 3: 1:45 pm - 2:45 pm/ Poster #9
BIO
I am a senior at FSU majoring in Biology with a minor in Chemistry. I am also on the pre-medical track and will be applying this cycle. I have been doing research in Dr. Chase's Lab for 6 semesters and this is my second time presenting at the FSU research symposium! At FSU, I am the treasurer of Alpha Epsilon Delta, member of Phi Beta Kappa, and a Captain in Dance Marathon. I am a Certified Medical Assistant and interned at Patients First as well as abroad at Clinica Sorolla. During my gap year I plan to work as a Medical Assistant, continue research, and volunteer.
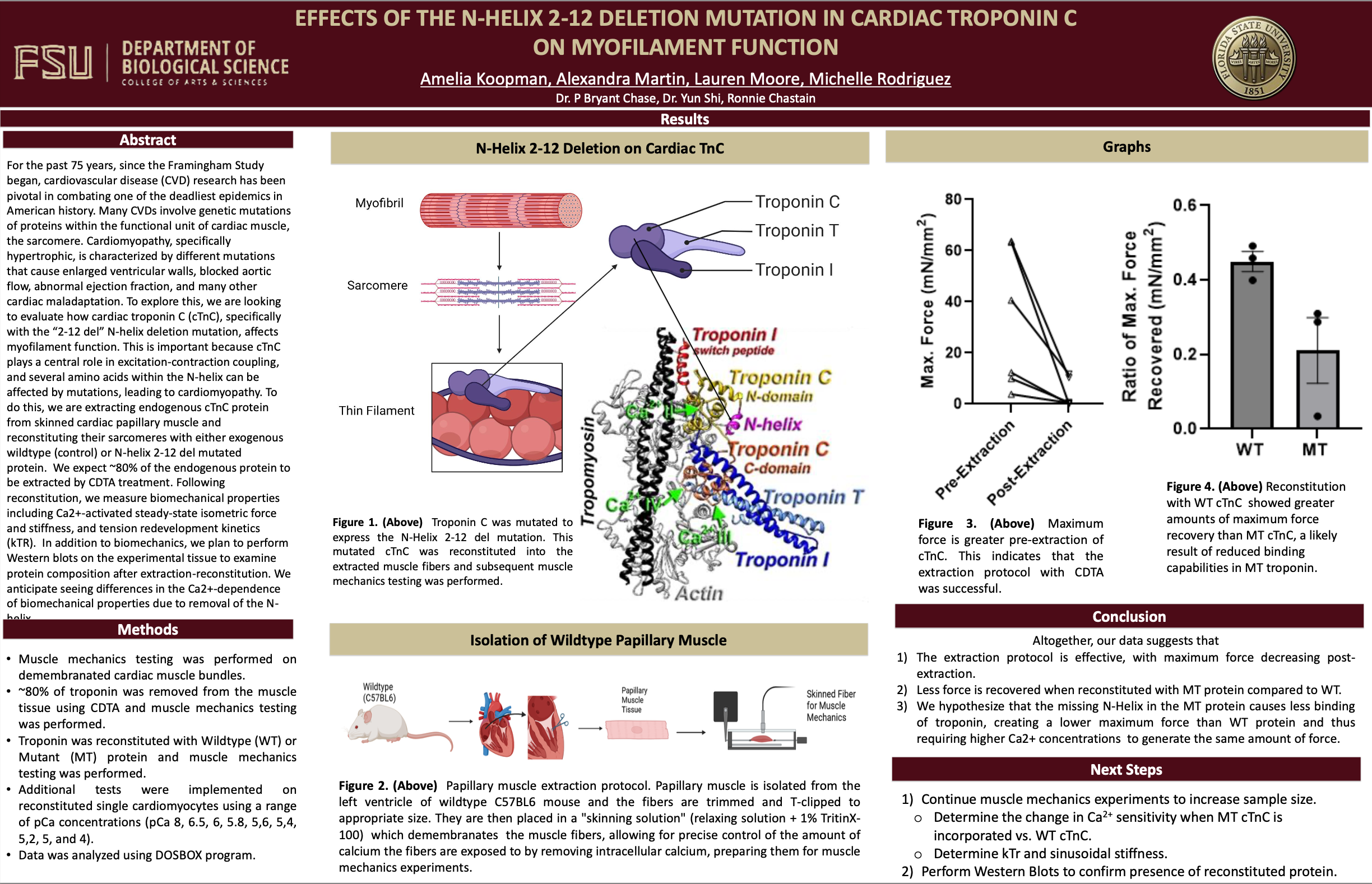
Effects of the N-Helix 2-12 Deletion Mutation in Cardiac Troponin C on Myofilament Function
Authors: Alexandra Martin, Dr. P. Bryant ChaseStudent Major: Biological Sciences
Mentor: Dr. P. Bryant Chase
Mentor's Department: Biological Science Mentor's College: College of Arts and Sciences Co-Presenters: Amelia Koopman, Lauren Moore, Michelle Rodriguez
Abstract
For the past 75 years, since the Framingham Study began, cardiovascular disease (CVD) research has been pivotal in combating one of the deadliest epidemics in American history. Many CVDs involve genetic mutations of proteins within the functional unit of cardiac muscle, the sarcomere. Cardiomyopathy, specifically hypertrophic, is characterized by different mutations that cause enlarged ventricular walls, blocked aortic flow, abnormal ejection fraction, and many other cardiac maladaptation. To explore this, we are looking to evaluate how cardiac troponin C (cTnC), specifically with the “2-12 del” N-helix deletion mutation, affects myofilament function. This is important because cTnC plays a central role in excitation-contraction coupling, and several amino acids within the N-helix can be affected by mutations, leading to cardiomyopathy. To do this, we are extracting endogenous cTnC protein from skinned cardiac papillary muscle and reconstituting their sarcomeres with either exogenous wildtype (control) or N-helix 2-12 del mutated protein. We expect ~80% of the endogenous protein to be extracted by CDTA treatment. Following reconstitution, we measure biomechanical properties including Ca2+-activated steady-state isometric force and stiffness, and tension redevelopment kinetics (kTR). In addition to biomechanics, we plan to perform Western blots on the experimental tissue to examine protein composition after extraction-reconstitution. We anticipate seeing differences in the Ca2+-dependence of biomechanical properties due to removal of the N-helix.
Keywords: Troponin, Cardio Myopathy, Mutations, Muscle Mechanics.