Research Symposium
24th annual Undergraduate Research Symposium, April 3, 2024
Lazaro Castano Poster Session 5: 4:00 pm - 5:00 pm/251

BIO
I'm a 2nd year biomedical engineering student from Naples, Florida. Throughout my life, I've wanted to make a difference, so through the combination of engineering and biology, I hope to do just that.
Structural Characterization Of Lupus Antigen-Related Proteins With Solution-State NMR & Electron Microscopy
Authors: Lazaro Castano, Nolan BlackfordStudent Major: Biomedical Engineering
Mentor: Nolan Blackford
Mentor's Department: Biochemistry Mentor's College: Biochemistry Co-Presenters:
Abstract
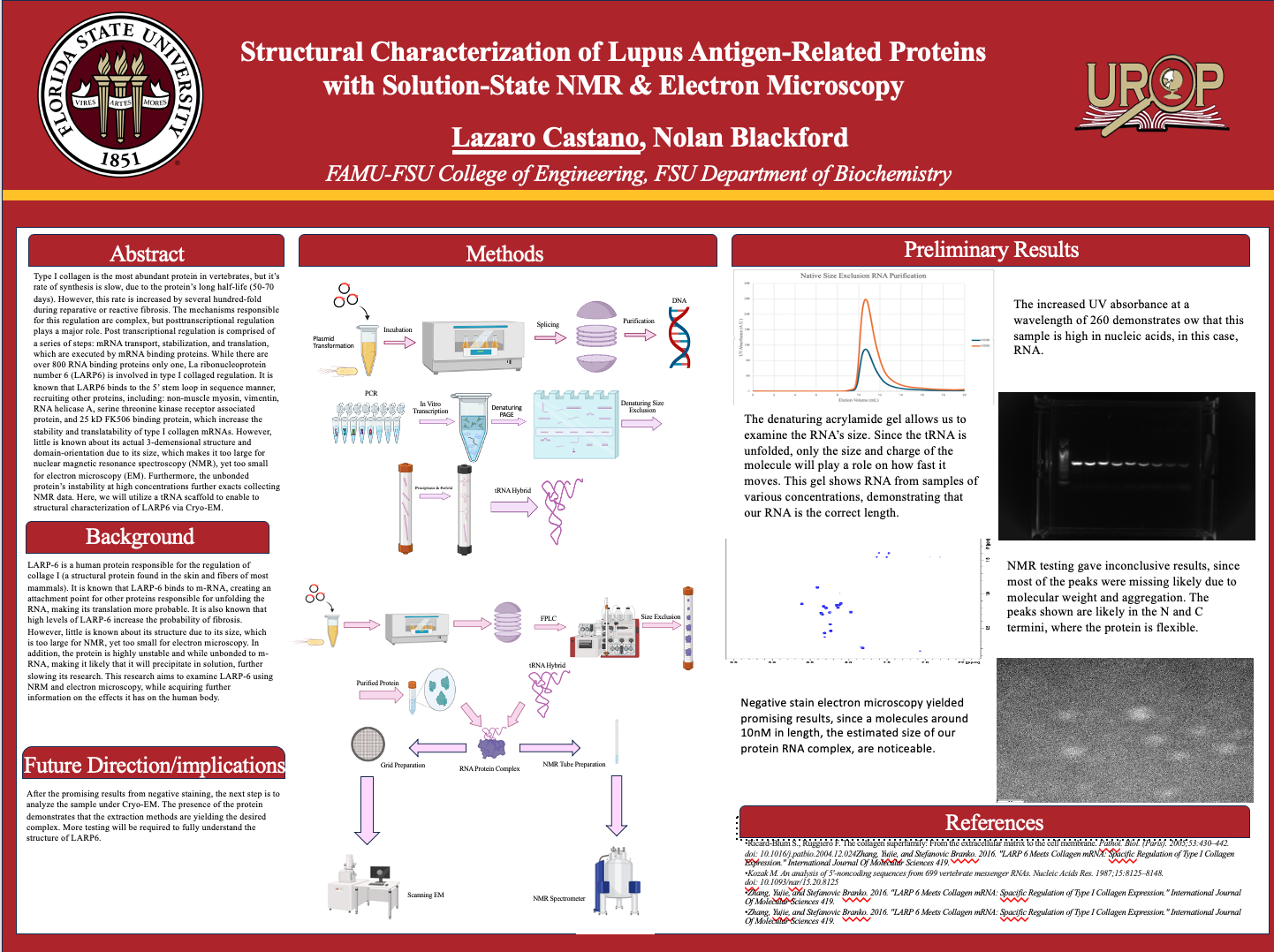
Type I collagen is the most abundant protein in vertebrates, but it’s rate of synthesis is slow, due to the protein’s long half-life (50-70 days) [1]. However, this rate is increased by several hundred-fold during reparative or reactive fibrosis. The mechanisms responsible for this regulation are complex, but posttranscriptional regulation plays a major role. Post transcriptional regulation is comprised of a series of steps: mRNA transport, stabilization, and translation, which are executed by mRNA binding proteins. While there are over 800 RNA binding proteins only one, La ribonucleoprotein number 6 (LARP6) is involved in type I collaged regulation [2]. It is known that LARP6 binds to the 5’ stem loop in sequence manner, recruiting other proteins, including: non-muscle myosin, vimentin, RNA helicase A, serine threonine kinase receptor associated protein, and 25 kD FK506 binding protein, which increase the stability and translatability of type I collagen mRNAs [3]. It is also known that high levels of LARP6 contribute to fibrotic disease [4]. However, little is known about LARP6’s 3-demensional structure and domain-orientation due to its size, which makes it too large for nuclear magnetic resonance spectroscopy (NMR), yet too small for electron microscopy (EM). Furthermore, the unbonded protein’s instability at high concentrations further exacts collecting NMR data. Here, we will utilize a tRNA scaffold to enable structural characterization of LARP6 via Cryo-EM. Furthermore, we will reexamine the protein using NMR spectroscopy attempting previously failed techniques.
Keywords: LARP6, NMR, Protein Structural Characterization