Research Symposium
24th annual Undergraduate Research Symposium, April 3, 2024
Cristina Dabrowski Poster Session 4: 2:45 pm - 3:45 pm /58

BIO
Cristina Dabrowski is a 4th year Biological Sciences and Music dual degree student from Tampa, Florida. She is currently conducting her Honor’s Thesis research in the Yin Lab at Florida State University. After graduation, she plans to pursue a career in academic medicine, conducting research in the field of immunology.
Characterization of Guanylate Binding Protein 2
Authors: Cristina Dabrowski, Dr. Qian YinStudent Major: Biological Sciences and Music
Mentor: Dr. Qian Yin
Mentor's Department: Department of Biological Sciences Mentor's College: College of Arts and Sciences Co-Presenters:
Abstract
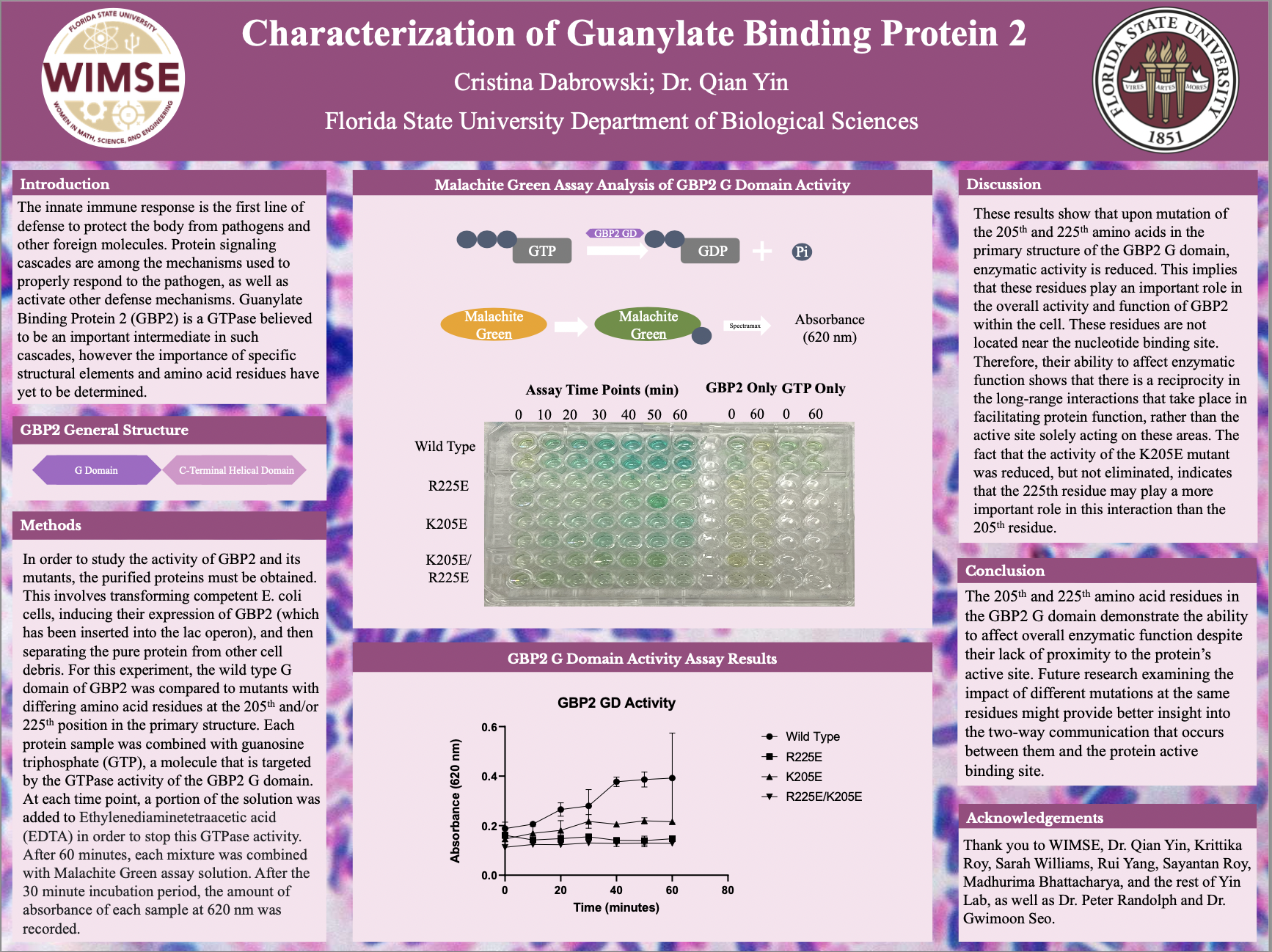
While the adaptive immune response begins to effectively combat pathogens several days after first infection, the pathways that comprise the innate immune response allow the host organism to immediately begin neutralizing foreign bodies. Guanylate Binding Protein 2 (GBP2) is a GTPase protein involved with such pathways, contributing specifically to cell-autonomous innate immunity. This project investigates the potential impact of amino acid residues outside of the protein’s active site on substrate binding, in order to better understand the specific mechanisms and regulatory methods through which GBP2 contributes to innate immunity. Specifically, the G domain of wild-type GBP2 (GBP2 GD) was purified along with proteins containing mutations in the 205th and 225th amino acid positions to observe the impact of these residues on overall enzymatic activity. This was accomplished by transforming competent E. coli cells, inducing protein expression, lysing the cells, and then using affinity purification ion-exchange and size-exclusion chromatography to isolate the purified proteins. These protein samples were then introduced to a buffer solution containing guanosine triphosphate (GTP). Activation levels were then measured by performing a Malachite Green assay to determine the amount of GTP hydrolyzed at each time point (10-minute intervals for 60 minutes). Enzymatic activity was reduced in all mutants and was eliminated in GBP2 samples containing the R225E and K205E/R225E mutations. This implies that the residues in the 205th and 225th positions demonstrate the ability to affect overall enzymatic function despite their lack of proximity to the protein’s active site.
Keywords: protein, immunology, molecular biology