Research Symposium
24th annual Undergraduate Research Symposium, April 3, 2024
Victoria Montalvo Poster Session 3: 1:30 pm - 2:30 pm/222
BIO
My name is Victoria Adelina Montalvo (preferred name Vicky) and I am a first year student pursuing Cell and Molecular Neuroscience Degree with a minor in Sociology at Florida State University. I am from Miami, Florida and am currently on the pre-medical track, with aims to attend medical school and become a doctor. My vast research interests include but are not limited to: microbiology, prions, cognitive psychology, eating disorder studies, space medicine, ophthalmology, molecular cloning, microsociology, and foreign language study.
Protein engineering of alpha-actinin mutant proteins to test biological role in the heart muscle
Authors: Victoria Montalvo, Christopher Solis, PhD, MBAStudent Major: Cell and Molecular Neuroscience
Mentor: Christopher Solis, PhD, MBA
Mentor's Department: Health, Nutrition, and Food Sciences Mentor's College: National University of Costa Rica, University of Illinois at Chicago, South Dakota State University Co-Presenters: Jenny Harper, Claudia Hernandez
Abstract
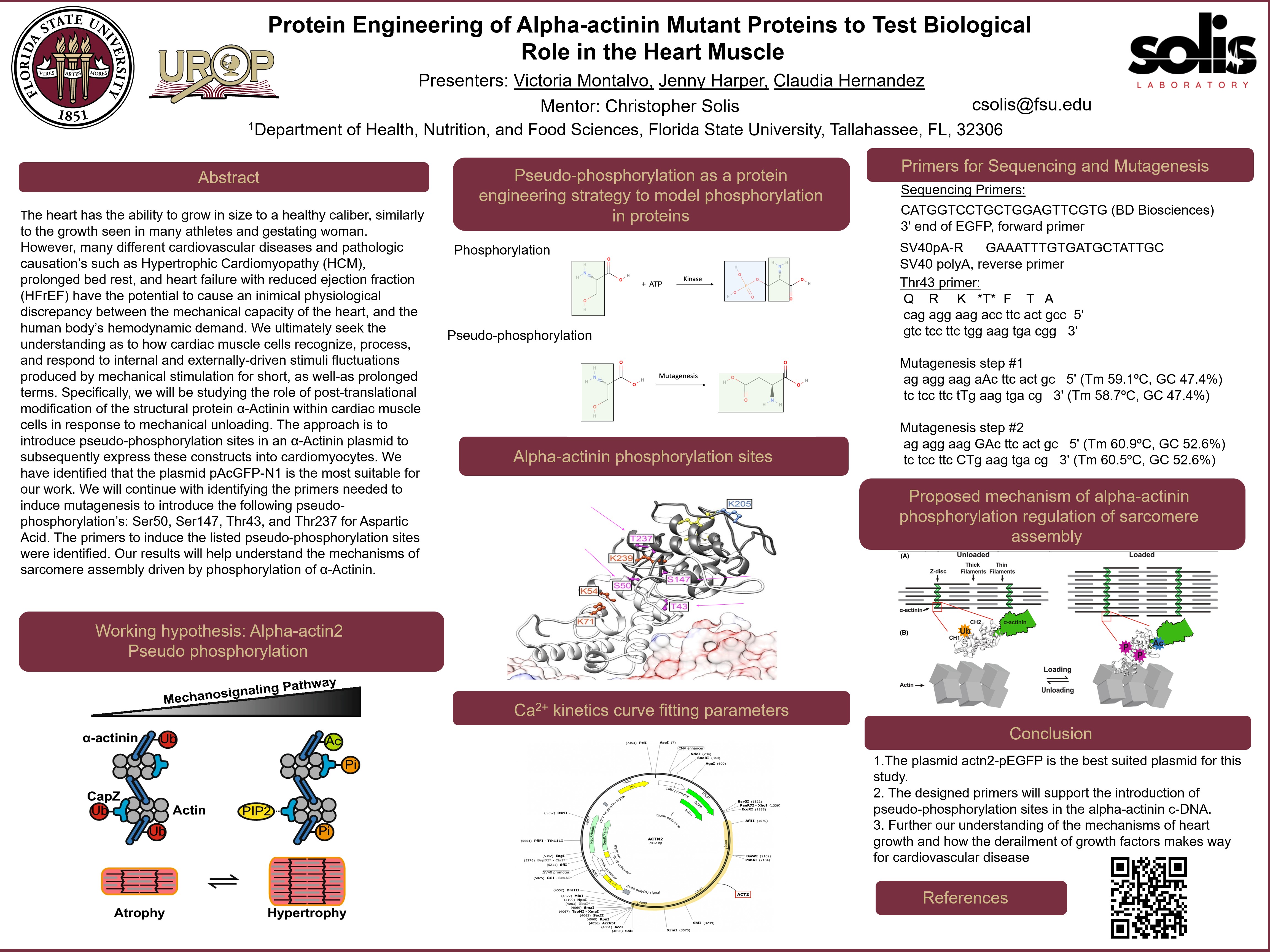
The heart has the ability to grow in size to a healthy caliber, similarly to the growth seen in many athletes and gestating woman. However, many different cardiovascular diseases and pathologic causation’s such as Hypertrophic Cardiomyopathy (HCM), prolonged bed rest, and heart failure with reduced ejection fraction (HFrEF) have the potential to cause an inimical physiological discrepancy between the mechanical capacity of the heart, and the human body’s hemodynamic demand. We ultimately seek the understanding as to how cardiac muscle cells recognize, process, and respond to internal and externally-driven stimuli fluctuations produced by mechanical stimulation for short, as well-as prolonged terms. Specifically, we will be studying the role of post-translational modification of the structural protein α-Actinin within cardiac muscle cells in response to mechanical unloading. The approach is to introduce pseudo-phosphorylation sites in an α-Actinin plasmid to subsequently express these constructs into cardiomyocytes. We have identified that the plasmid pAcGFP-N1 is the most suitable for our work. We will continue with identifying the primers needed to induce mutagenesis to introduce the following pseudo-phosphorylation’s: Ser50, Ser147, Thr43, and Thr237 for Aspartic Acid. The primers to induce the listed pseudo-phosphorylation sites were identified. Our results will help understand the mechanisms of sarcomere assembly driven by phosphorylation of α-Actinin.
Keywords: Cardiac, Plasmids, Molecular Cloning, Cardiovascular, Muscle, Cell growth